Podcast: Artificial photosynthesis for sustainable solar fuels
In S1E1, Prof. Zetian Mi talks unlocking quantum properties to close the loop on carbon emissions.
Even after COVID-19 is controlled, climate change, growing resistance to antibiotics and lack of clean water will still be waiting for us. We need research that could change… everything. That’s what Blue Sky is all about.
What if we could learn from the trees to make a fossil fuel alternative out of carbon dioxide and sunlight alone? We could close the loop on carbon emissions—a critical win in the battle against climate change. That’s one of the goals of Zetian Mi, professor of electrical engineering and computer science. He’s also aiming to make efficient, affordable ultraviolet LEDs to sterilize drinking water in parts of the world that don’t have enough today. Connecting these two technologies is an up-and-coming semiconductor called gallium nitride.They think it may be capable of guiding energy transitions that are too powerful for ordinary semiconductors like silicon. Can Mi’s team engineer the quantum properties so that gallium nitride lives up to its promise?
About the podcast: The Blue Sky podcast is a limited series from RE: Engineering Radio and the University of Michigan College of Engineering. It delves into the four research projects funded as part of the $6 million Blue Sky initiative. Launched in 2018, the initiative gives research teams the freedom to try daring ideas, show results and build momentum to secure further research investment in their efforts to solve global problems. Season 1 is an introduction to each project.
Transcript
[Opening banter]
Nicole: Welcome to the Blue Sky Podcast. Today, we’ll talk to an engineering professor who’s working on artificial photosynthesis that’s up to 100 times more efficient than plants. This kind of research could lead to clean solar fuels that replace fossil fuels. Because right now, most of the way we produce energy ends up damaging the planet, or at least making it less hospitable to humans. It doesn’t have to be that way. I’m Nicole Casal Moore.
Jim: I’m Jim Lynch. and I’ve got to say my money in this situation is on the plants. You said 100 times more efficient?
 Enlarge
Enlarge
Nicole: Yeah, 100 times.
Jim: We’re talking about the single most important chemical process on Earth. Photosynthesis literally made this planet capable of supporting human life. And plants have had billions of years to perfect that.
Nicole: I know, but clever people have been trying for decades to reverse engineer it. It could, like, solve climate change.
Jim: High risk and high reward—I get that. It doesn’t change my bet though.
Nicole: I know it’s a long shot, Jim, but let’s hear from Professor Zetian Mi. I kind of called him on this one.
Nicole: Forgive me if this sounds insulting, but what makes you think you all can do this better than nature?
Jim: Did you really ask him that?
Nicole: I did.
Jim: Was he insulted?
Nicole: I don’t think so.
=====
Zetian Mi: That’s a great question. So what’s really good about semiconductor technology is this scalability. Think about computers. If you go back to the 1960s and 1970s, the computers at that time, you know, occupied the entire room. And look at today what we have. Look at solar cells. Forty years ago, could we envision solar cells will be everywhere producing electricity? Artificial photosynthesis is, by far, much more complicated than these other devices. But once we understand this process, then based on our history, there’s no reason we cannot make it better than nature.
=====
Jim: You’re gonna have to break that one down a little. Start with the semiconductor.
Nicole: Okay. The main thing to know about semiconductors is that they conduct electricity, but only under certain circumstances. You have to give some of their electrons a boost in energy so that they break free of their atoms, and jump into what’s called the conduction band.
Jim: That’s the stream of free electrons available for us to harness for electricity.
Nicole: Yeah.
Jim: But again, how do they connect artificial photosynthesis to solar cells, or computers?
Nicole: Well, semiconductors are at the core of all these technologies. And what Zetian is saying is that once you nail down the semiconductor, it’s not hard to scale it. That’s why he doesn’t think it’s impossible to do so much better than trees.
Jim: But solar cells turn sunlight into electricity. How does that relate to artificial photosynthesis? Plants turn sunlight, water, and carbon dioxide into sugars for food, and, I mean, isn’t that a lot?
Nicole: You can think of solar cells as doing the first step. And I’m about to get dense here. Just a warning. The solar cells take a photon, which is a particle of light from the Sun, and they use it to kick an electron into that conduction band where it can move around. We use the electrons directly, generating electrical currents. Sunlight does the same thing in plants, but the plants take that free electron and they do something else with it. They use it to spark a chemical reaction that makes the sugars that they feed on.
Jim: So Professor Mi wants to take that next step and move on to the chemical reaction.
Nicole: Exactly. Listen to this:
=====
Nicole: Talk about what this would look like if your technology works, and you’re able to achieve this. These artificial photosynthesis “plants”—would they be in cars? Would they be… powering our homes?
Zetian Mi: Well, if we are successful, I can give you two examples. So one example is, instead of a solar farm, we have the solar refinery plants that will produce chemical fuels instead of electricity, with the inputs being carbon dioxide and water.
Nicole: Where would the solar fuels be useful?
Zetian Mi: The solar fuel is essentially similar to the fossil fuels. You can use them, for example, to drive a car, or any other stuff. You can power cities, homes, factories.
Nicole: Could it replace our entire fossil fuel…?
Zetian Mi: Yeah. I think if we are successful, they replace fossil fuels.
=====
Jim: Right, because fossil fuels are made out of the same carbon and hydrogen as sugars. Professor Mi’s team can just put them together differently and—bam—right? Solar refinery?
Nicole: It’s that simple! No, it’s quite difficult, but yes, that’s the idea. Believe it or not, that’s not all he’s doing in this.
Jim: So one grand challenge wasn’t enough for him?
Nicole: Apparently not. He’s also working on an ultraviolet light to purify water.
=====
Zetian Mi: In many places in China, people do not have access to clean drinking water. China’s not alone. There are nearly 2 billion people who do not have access to clean water, and that bothers me. The way we purify water, mostly we use chemical treatment. Chemical treatment works well, but you have residue.
=====
Jim: Did he say residue in my water?
Nicole: It’s just the chlorine leftover from killing the bacteria.
=====
Zetian Mi: If we use ultraviolet lights, it will be a very simple process, a very clean process.
=====
Nicole: So the light’s so powerful that it kills the bacteria without needing any chemicals.
Jim: You clean the water by running sewage past this ultraviolet light?
Nicole: Well, you still need the earlier steps that remove things like sand and sludge, but instead of just dumping some chlorine in the water to get rid of the bacteria, you use the UV light, and no chlorine odor in your city water.
Jim: And no cholera. Save the chlorine for the kids’ pool, I say. All right, so these still sound like two totally different projects.
Nicole: They both rely on high-performance semiconductors that don’t yet exist.
Jim: Those semiconductors—right. We’ve covered that. Those are the things that conduct electricity when you give their electrons a little juice?
Nicole: Yes. I have a question for you. Have you heard of gallium nitride?
Jim: No.
Nicole: I hadn’t either.
=====
Nicole: Okay, is it gallium nitride?
Zetian Mi: Yes.
Nicole: Okay. Not nitrate?
Zetian Mi: Gallium nitride is a semiconductor that has indium, gallium, aluminum, with nitrogen.
=====
Jim: Sounds like my Friday night shopping list.
=====
Zetian Mi: It’s everywhere. You probably didn’t realize that. The material for an LED light bulb is gallium nitride. Also gallium nitride has been widely used in ultraviolet light emitting devices.
=====
Jim: Gallium nitride.
Nicole: It’s so hot right now.
Jim: Zoolander reference. I got that.
Nicole: Here’s a headline in the Verge from February, 2019. “Gallium nitride is the silicon of the future.” The subhead actually said, “Gallium Nitride Valley,” like instead of Silicon Valley. Now, this is the semiconductor that’s behind Anker’s Power Brick technology. I don’t know if you have any of these. They’re for computers and smartphones. And the cool thing about them is that they charge really fast. This is not a semiconductor that is relegated to just research labs. UV photons are very high energy. So to get a high energy photon, you need to start with the high energy charge carriers inside the semiconductor.
Jim: The LED is sort of the reverse of a solar cell. Instead of taking light and turning it into an electric current, you’re taking an electric current, turning it into light, right?
Nicole: Exactly. That’s a cool way to look at it. And to get that UV photon, you need that current to produce high energy charge carriers. When I say charge carriers, let me clarify. I’m talking about negatively charged electrons and positively charged holes.
Jim: Is that a serious thing?
Nicole: Yeah. Yeah. They’re a thing. Holes are the spaces left behind by the electrons. Because the electron is gone, there’s this positive charge left behind in the lattice of atoms.
Jim: Lattice of atoms.
Nicole: When an electron and hole come together, that produces a photon—a particle of light. And that’s how an LED works.
Jim: Does that make an LED a quantum device?
Nicole: Yeah.
Jim: And if you have enough LEDs, that can teleport me through time, perhaps?
Nicole: We don’t know that yet. We don’t know what else Professor Mi’s working on, but it’s not part of this.
Jim: So in the solar refinery, we’ve got photons coming in, creating electrons in these holes you’re talking about.
Nicole: And then Zetian’s team is going to design a chemical reaction that will use those high energy electrons and holes to make a fuel.
Jim: Okay, I get that we already have LED lights and solar panels, why don’t we have UV LEDs and solar refineries?
Nicole: The central problem that Professor Mi’s team is trying to solve is how to control those electrons, holes, and photons in a way that concentrates the energy.
Jim: It’s like trying to get everyone at the wedding on the dance floor to start that electric slide.
Nicole: That concentrated energy—you need an electron and a hole that are each carrying a lot of energy to come together in a particular way if you want to pour all that energy into making a bacteria-killing UV photon. Today’s semi-conductors are more likely to lose that energy as heat.
=====
Zetian Mi: We need to deal with the very tiny particles—electrons, holes, and photons—in a way that they can interact very, very efficiently. This requires very careful quantum engineering.
=====
Jim: All right, to get this artificial photosynthesis dance party going, we’re going to need to concentrate more energy in the electrons and the holes that are produced by the semiconductor. Do I have that right?
Nicole: You got it.
Jim: At least compared to what we’ve been doing with solar cells previously.
Nicole: So chlorophyll in plants is actually better at concentrating solar energy than we are, which makes sense. They have one job.
Jim: You’re saying that my house plant would be considered a quantum device?
Nicole: It really is.
Jim: Well, they’re artificial houseplants.
Nicole: Ha, they’re still kind of quantum, even though they aren’t harnessing quantum mechanics for photosynthesis.
Jim: So what is going to make these electrons drunk enough to get on that dance floor and do some artificial photosynthesis?
Nicole: Well, your semiconductor has to be designed so that the electrons and holes have to jump over this high hurdle before they could even get to the dance floor. This makes sure that the charge carriers, those electrons and holes, all have enough energy to do what you want them to do.
Jim: Every DJ knows this. You make them jump to increase the energy. So what’s the problem here?
Nicole: Well, if the hurdle is too high, you don’t get many charged carriers moving around, but if it’s too low, they don’t have enough energy to make a UV photon. They’ll just make some other kind of light.
Jim: How are they gonna fix that?
Nicole: Professor Mi said that they’ll start by growing very high quality aluminum gallium nitride.
=====
Nicole: Now, where is… I assume there’s some gallium nitride around here?
Zetian Mi: Ah, yes.
Nicole: Where is that?
Zetian Mi: Oh, you mean the materials?
Nicole: Yeah, the material. Like, I’d love for us to see it.
Zetian Mi: Take a look?
Nicole: Yeah.
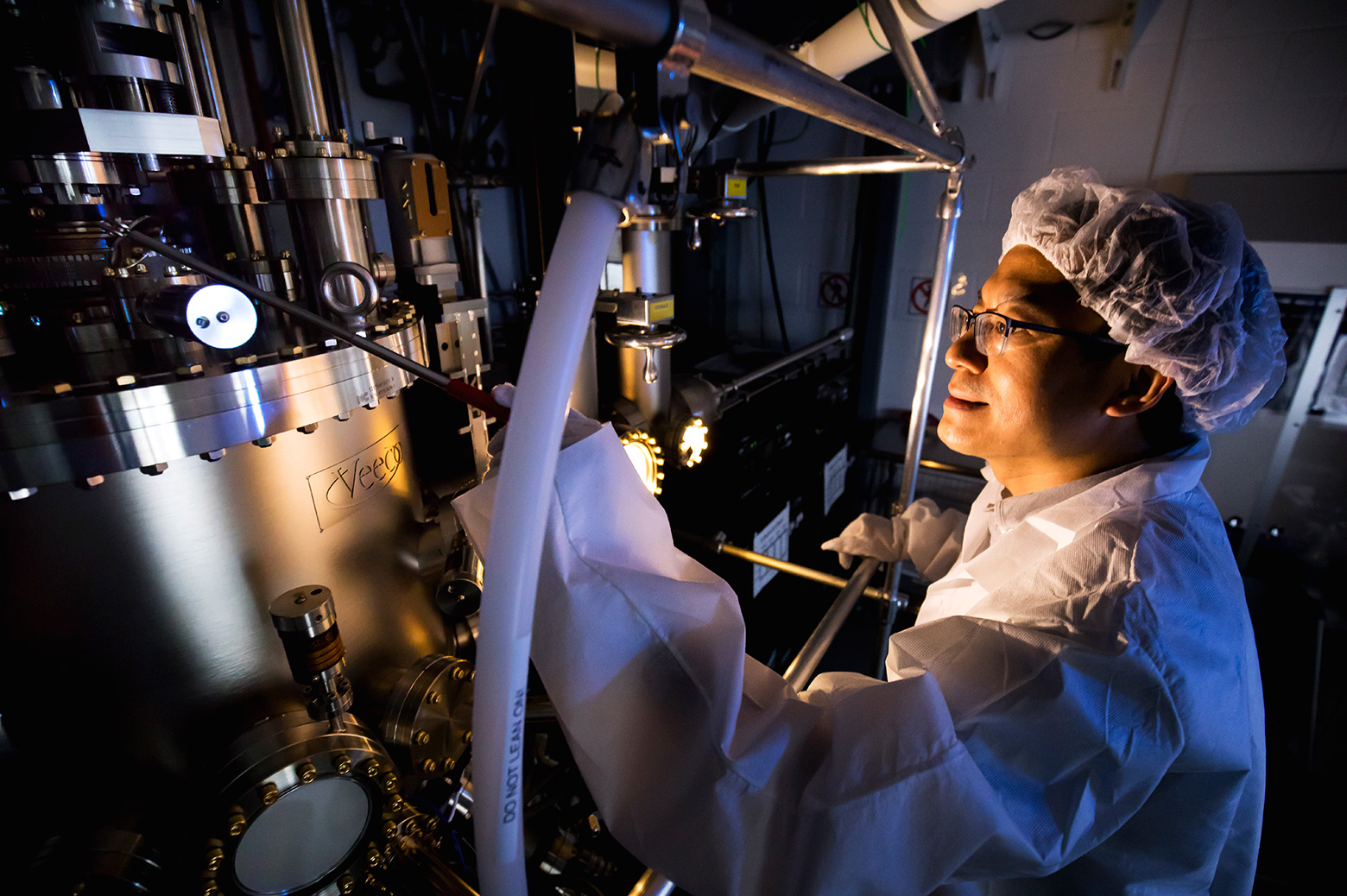
Zetian Mi: I can show you in the lab. We’re going one more floor, yeah. This is the machine where we grow the materials.
=====
Jim: You grow nitride? Aluminum, gallium, whatever you call that, you grow it?
Nicole: Well, I don’t. But Professor Mi does.
=====
Nicole: So Zetian tell us where we are right now.
Zetian Mi: So we are in the MBE lab.
=====
Jim: M-B-E lab?
Nicole: Molecular Beam Epitaxy. It’s a fancy way of saying it’s a lab where they arrange atoms in very thin layers on top of each other.
Jim: You sound like you’re describing atomic phyllo dough.
Nicole: I like that.
Jim: Fee-lo. Figh-llo.
Nicole: So with your atomic phyllo dough, you can grow very precise semiconductor crystals. So they heat up gallium, nitrogen and the rest of your Friday night shopping list.
Jim: Indium, gallium, aluminum, nitrogen.
Nicole: And they shoot them at a wafer. It can be a piece of silicon, or often with gallium nitride, it’s sapphire.
Jim: So they’re growing it on jewels?
Nicole: Artificial jewels, I guess. Scientists can make sapphire wafers, which are structured like the gem. Sapphire happens to have a structure that’s similar to gallium nitride, so it helps the gallium nitride form better crystals.
Jim: Okay, well then where do they bake their atomic phyllo dough?
Nicole: In the oddest metal chamber I have ever seen.
=====
Nicole: I’m actually not good at visually describing things. Large. I’m looking at a large metal apparatus with a lot of, what look like valves, and circular components. I’m gonna describe it as Dr. Suessian.
=====
Jim: Dr. Suessian?
Nicole: Yeah.
=====
Zetian Mi: Whenever we open the chamber, we need to bake the entire system to 200 degrees Celsius for a week. So everything attached to the system must be bakeable. They must survive up to 200 degrees Celsius for a few days.
=====
Jim: They really bake it?
Nicole: They bake it to clean it. Even though the machine is in a so-called clean room, there’s a lot of crud that comes in with the air whenever the machine is open. So they have to heat it way up to vaporize everything and pump as much air out as they can.
Jim: It’s like a self cleaning oven, right?
Nicole: Plus vacuum. It’s actually more empty than outer space when it’s running.
Jim: More empty than space. That’s pretty empty.
=====
Zetian Mi: In some regions, it will be very hot, because we are evaporating elements. In other regions it will be very cold, because we have flowing liquid nitrogen. And the reason we want cold is because we want that region to be very clean. The cold serves as a trap of the impurities.
Nicole: Where’s the gallium nitride?
Zetian Mi: So you can see the wafers here lying around.
Nicole: So there’s, okay, so these little squares here, these little gold looking squares are gallium nitride?
Zetian Mi: Yeah.
Nicole: They’re about the size of my pinky nail.
Zetian Mi: The material, the actual material that we are talking about is actually very thin. It’s only on the order of a few hundred nanometers. And then once we have the wafers, then we can go to the Lurie Nanofabrication Facility to make the devices, and then do the testing in other labs.
=====
Jim: It sounds like they’re at square one right now, just making the materials. Are they playing with the chemistry, trying to get enough charge carriers?
Nicole: Yes. That’s what they’re doing at this stage. Earlier on, when he was saying what was in the semiconductors, it wasn’t just gallium and nitrogen.
Jim: Indium, gallium, aluminum, nitrogen.
Nicole: You remember.
Jim: I can do this all day.
Nicole: So there are actually two more atoms that can go in: silicon to add electrons to the gallium nitride, or magnesium to add holes. These are called dopants.
Jim: Like sports drugs for semiconductors.
Nicole: You could say that. They do enhance the performance. You could say they add charge carriers with fewer inhibitions.
Jim: Who will get on the quantum engineer dance floor.
Nicole: Yes. That’s what the silicon and magnesium do. But then you have to add indium to make the hurdle lower. That’s what you get in a blue LED, or, what Professor Mi and his team are doing, you add aluminum to make the hurdle higher. Problem is, once you do that, it’s especially hard to form those holes.
Jim: So what these semiconductors need are some, well, the way we’re putting it, stronger drugs?
Nicole: In a way, I guess, but that is the chemistry challenge. Create a semiconductor that can support a lot of high energy charged carriers for both of the applications that Professor Mi is trying to do here. And once they do that, they can turn the charge carrier into UV photons for water purification, or use the energy for artificial photosynthesis and chemical processing in a solar refinery.
Jim: This is something they think they can get to inside of three years in the Blue Sky program?
Nicole: Well, I asked about that.
=====
Nicole: How likely are you to succeed here? I know part of the thinking behind the Blue Sky Initiative is to pick really hard problems that you might fail at solving.
Zetian Mi: Well, we did the first step, right? We think of the very hard problems. I think if we can bring this to the level of commercialization in two to three years, then it will be a tremendous success. Realistically speaking, we may not be able to bring this to commercialization entirely. I think we should be able to bring this to a level that will attract significant attention. Then we will get significant investment and support from federal funding agencies, from industry, or nonprofit organizations.
=====
Jim: So he thinks his team can take this problem from a way out, hard-to-get-funding type of situation, to something that is seen as an investment opportunity?
Nicole: That’s his plan.
Jim: Nicole, I’m looking forward to hearing back from you after some time to see if the good professor and his Blue Sky team have found the right DJ mix to keep the electron and hole party going strong into the AM. I’m Jim Lynch, and this is the Blue Sky Podcast.

 MENU
MENU